Andre Beringhs (UConn), Bruna Minatovicz (UConn), Geoff Zhang (CPPR IAB from Abbvie), Bodhi Chaudhuri (UConn), and Xiuling Lu published their CPPR research findings on “Impact of Porous Excipients on the Manufacturability and Product Performance of Solid Self-Emulsifying Drug Delivery Systems” in AAPS PharmSci Tech, 19(7):3298-3310 (2018). 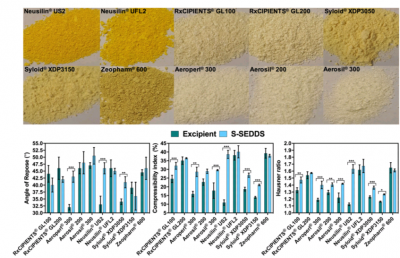
Shreya Kulkarni awarded Baxter Young Investigator

Shreya Kulkarni, a doctoral candidate in the Bogner lab at UConn, was awarded one of six Tier I Baxter Young Investigator awards at the headquarters of Baxter in Illinois in October 2018.
CPPR Project Reveals Nanometer-Scale Residual Crystals in Hot Melt Extrudates
Dana Moseson, graduate student in Lynne Taylor’s lab at Purdue University describe the characterization of residual crystallinity in hot melt extrudates down to the nanometer-scale using a suite of techniques. See Moseson DE, Mugheirbi NA, Stewart AA, Taylor LS. Nanometer-Scale Residual Crystals in a Hot Melt Extruded Amorphous Solid Dispersion: Characterization by Transmission Electron Microscopy. Crystal Growth & Design. 2018 Oct 26;18(12):7633-40.

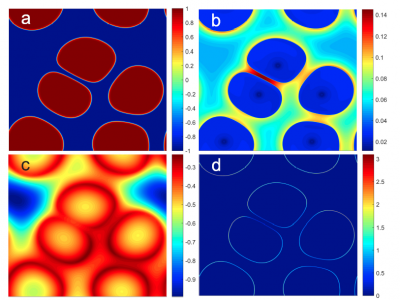
Publication of CPPR Research – Modeling of Freeze Concentration
Professor Tai-Hsi Fan (UConn Dept of Mechanical Engineering) along with colleagues at the UConn School of Pharmacy, Regeneron Pharmaceuticals, and MedImunne published an article describing “Phase-Field Modeling of Freeze Concentration of Protein Solutions” in the journal, Polymers (2019) Vol. 11, article 10.
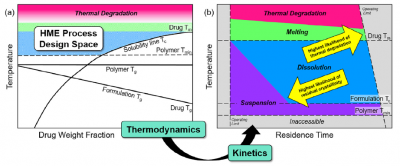
Hot Melt Extrusion Publication from CPPR
Dana Moseson and her advisor Lynne Taylor from Purdue University published their CPPR-funded work on applying phase diagrams to prevent residual crystallinity in hot melt extrudates. See Moseson DE, Taylor LS. The application of temperature-composition phase diagrams for hot melt extrusion processing of amorphous solid dispersions to prevent residual crystallinity. International journal of pharmaceutics. 2018 Dec 20;553(1-2):454-66.
New CPPR Research Publication
Shreya Kulkarni (UConn), Raj Suryanarayanan (UMinnesota), Joe Rinella (CPPR IAB Mentor from GSK) and Robin Bogner (UConn) published their CPPR research findings on “Mechanisms by which crystalline mannitol improves the reconstitution time of high concentration lyophilized protein formulations” in European Journal of Pharmaceutics and Biopharmaceutics, 131, 70-81 (2018).

Raj Sury student, Kweku Amponsah-Efah, wins IPEC Award
Kweku Amponsah-Efah, grad student at the University of Minnesota, in Dr. Raj Suryanarayanan’s lab, received one of the 2018 International Pharmaceutical Excipients Council Foundation Graduate Student Scholarship Awards. The award was presented at the IPEC foundation dinner, at the 2018 AAPS annual meeting in Washington, D.C. on Nov. 4th. Kweku presented two posters: “Characterizing the nature of drug-polymer complexes in aqueous solution using analytical ultracentrifugation” and “Probing the temperature-dependence of structural relaxation times deep in the glassy state of amorphous pharmaceuticals.”

Burgess receives AAPS Wurster Research Award
Diane Burgess (UConn) received the AAPS Dale E. Wurster Research Award in Pharmaceutics at AAPS PharmSci360, November 2018 in Washington, DC. For more, see the article in UConn Today.

Stephen R. Byrn Recognized by the AAPS
Professor Stephen R. Byrn (Purdue University) was recognized by the American Association of Pharmaceutical Scientists with the AAPS Pharmaceutical Global Health Award. Dr. Byrn is one of the cofounders of the Kilimanjaro Sustainable Medicines in Africa Program, which is dedicated to enabling quality manufacturing of critical medicines in Africa by Africans.

See the video describing the award.
CPPR Findings Published
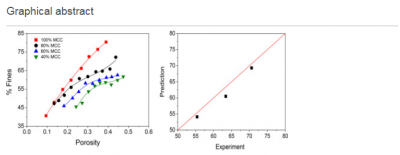
Wei-Jhe Sun (University of Minnesota), Jukka Rantanen and Calvin Sun (University of Minnesota) published their CPPR research findings on “Ribbon density and milling parameters that determine fines fraction in dry granulation” in Powder Technology, 338:162-167 (2018).